FDA Approves New Controversial Alzheimer’s Drug
TEHRAN (Tasnim) – The US Food and Drug Administration (FDA) granted conditional approval to a new drug, aducanumab, to be marketed as Aduhelm, for the treatment of Alzheimer’s disease.
US health officials on Monday approved the new drug for Alzheimer’s disease – the first in nearly 20 years – giving hope to 6.2 million Americans and many more worldwide over the age of 65 who suffer from the brain disease, Al Jazeera reported.
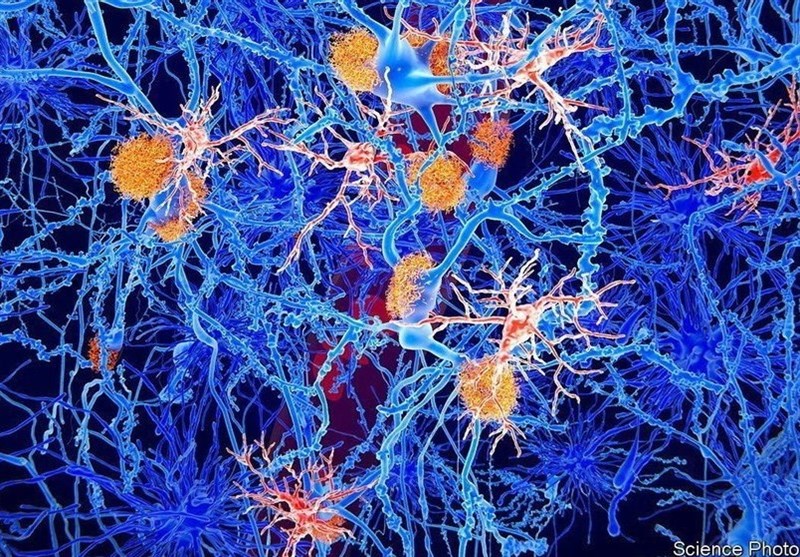
It is the only drug approved by US regulators to treat the underlying cause of the disease, the build-up of fatty plaque in the brain. Clinical trials of the new drug, developed by Biogen Inc, showed a reduction in the plaque, thus helping to slow mental decline.
However, the FDA’s approval of the drug is controversial because of warnings from independent experts that the treatment does not work. The FDA is requiring Biogen to conduct additional clinical trials to verify the drug’s expected benefits.
Groups representing Alzheimer’s patients and their families said any new therapy – even one of small benefit – warranted approval.
Biogen did not immediately disclose the price, though analysts have estimated the drug could cost between $30,000 and $50,000 for a year’s worth of treatment.
A preliminary analysis by the non-profit Institute for Clinical and Economic Review found that the drug would need to be priced at $2,500 to $8,300 a year to be a good value based on the “small overall health gains” suggested by company studies.
The new medicine is made from living cells that will have to be given via infusion at a doctor’s office or hospital. The Alzheimer’s Association hailed the decision as a historic step forward.
Researchers do not fully understand what causes Alzheimer’s but there is broad agreement that the brain plaque targeted by aducanumab is just one factor.
Growing evidence suggests family history, education and chronic conditions, such as diabetes and heart disease, may all play a role.
“This is just one piece of the puzzle and I think all these other options need to be explored and amplified,” Dr Ronald Petersen, a Mayo Clinic dementia specialist who has consulted for Biogen and other drugmakers told AP.
In November, an FDA outside panel of neurological experts voted “no” to a series of questions on whether reanalysed data from a single study submitted by Biogen showed that the drug was effective.
Biogen had halted two studies of the drug in 2019 after disappointing results suggested aducanumab, marketed by Biogen as Aduhelm, would not meet its goal of slowing mental and functional decline in Alzheimer’s patients.